Abstract
The roots,
stems, leaves, and vascular circuitry of higher plants are responsible for
conveying the chemical signals that regulate growth and functions. From a
certain perspective, these features are analogous to the contacts,
interconnections, devices, and wires of discrete and integrated electronic
circuits. Although many attempts have been made to augment plant function with
electroactive materials, plants’ “circuitry” has never been directly merged
with electronics. We report analog and digital organic electronic circuits and
devices manufactured in living plants. The four key components of a circuit
have been achieved using the xylem, leaves, veins, and signals of the plant as
the template and integral part of the circuit elements and functions. With
integrated and distributed electronics in plants, one can envisage a range of
applications including precision recording and regulation of physiology, energy
harvesting from photosynthesis, and alternatives to genetic modification for
plant optimization.
Keywords
- organic bioelectronics
- conducting polymers
- plants
INTRODUCTION
The growth
and function of plants are powered by photosynthesis and are orchestrated by
hormones and nutrients that are further affected by environmental, physical,
and chemical stimuli. These signals are transported over long distances through
the xylem and phloem vascular circuits to selectively trigger, modulate, and
power processes throughout the organism (1) (see Fig. 1).
Rather than tapping into this vascular circuitry, artificial regulation of
plant processes is achieved today by exposing the plant to exogenously added
chemicals or through molecular genetic tools that are used to endogenously
change metabolism and signal transduction pathways in more or less refined ways
(2). However,
many long-standing questions in plant biology are left unanswered because of a
lack of technology that can precisely regulate plant functions locally and in
vivo. There is thus a need to record, address, and locally regulate isolated—or
connected—plant functions (even at the single-cell level) in a highly complex
and spatiotemporally resolved manner. Furthermore, many new opportunities will
arise from technology that harvests or regulates chemicals and energy within
plants. Specifically, an electronic technology leveraging the plant’s native
vascular circuitry promises new pathways to harvesting from photosynthesis and
other complex biochemical processes.
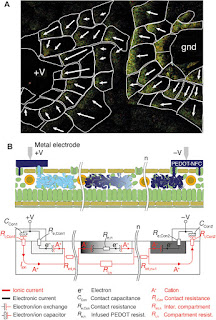
Fig. 1 Basic
plant physiology and analogy to electronics.
(A
and B) A plant (A), such as a rose, consists of roots, branches, leaves,
and flowers similar to (B) electrical circuits with contacts, interconnects,
wires, and devices. (C) Cross section of the rose leaf. (D)
Vascular system of the rose stem. (E) Chemical structures of PEDOT
derivatives used.
Organic
electronic materials are based on molecules and polymers that conduct and
process both electronic (electrons e−, holes h+) and
ionic (cations A+, anions B−) signals in a tightly
coupled fashion (3, 4). On the
basis of this coupling, one can build up circuits of organic electronic and
electrochemical devices that convert electronic addressing signals into highly
specific and complex delivery of chemicals (5), and vice
versa (6), to regulate
and sense various functions and processes in biology. Such “organic
bioelectronic” technology platforms are currently being explored in various medical
and sensor settings, such as drug delivery, regenerative medicine, neuronal
interconnects, and diagnostics. Organic electronic materials—amorphous or
ordered electronic and iontronic polymers and molecules—can be manufactured
into device systems that exhibit a unique combination of properties and can be
shaped into almost any form using soft and even living systems (7) as the
template (8). The
electronically conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) (9), either
doped with polystyrene sulfonate (PEDOT:PSS) or self-doped (10) via a
covalently attached anionic side group [for example, PEDOT-S:H (8)], is one of
the most studied and explored organic electronic materials (see Fig. 1E).
The various PEDOT material systems typically exhibit high combined electronic
and ionic conductivity in the hydrated state (11). PEDOT’s
electronic performance and characteristics are tightly coupled to charge
doping, where the electronically conducting and highly charged regions of PEDOT+
require compensation by anions, and the neutral regions of PEDOT0
are uncompensated. This “electrochemical” activity has been extensively
utilized as the principle of operation in various organic electrochemical
transistors (OECTs) (12), sensors (13), electrodes
(14),
supercapacitors (15), energy
conversion devices (16), and
electrochromic display (OECD) cells (9, 17).
PEDOT-based devices have furthermore excelled in regard to compatibility,
stability, and bioelectronic functionality when interfaced with cells, tissues,
and organs, especially as the translator between electronic and ionic (for
example, neurotransmitter) signals. PEDOT is also versatile from a circuit
fabrication point of view, because contacts, interconnects, wires, and devices,
all based on PEDOT:PSS, have been integrated into both digital and analog
circuits, exemplified by OECT-based logical NOR gates (18) and
OECT-driven large-area matrix-addressed OECD displays (17) (see Fig. 1B).
In the past,
artificial electroactive materials have been introduced and dispensed into
living plants. For instance, metal nanoparticles (19), nanotubes
(20), and
quantum dots (21) have been
applied to plant cells and the vascular systems (22) of
seedlings and/or mature plants to affect various properties and functions
related to growth, photosynthesis, and antifungal efficacy (23). However,
the complex internal structure of plants has never been used as a template for
in situ fabrication of electronic circuits. Given the versatility of organic
electronic materials—in terms of both fabrication and function—we investigated
introducing electronic functionality into plants by means of PEDOT.
RESULTS AND DISCUSSION
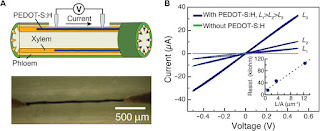
We chose to
use cuttings of Rosa floribunda (garden rose) as our model plant system.
The lower part of a rose stem was cut, and the fresh cross section was immersed
in an aqueous PEDOT-S:H solution for 24 to 48 hours (Fig. 2A),
during which time the PEDOT-S:H solution was taken up into the xylem vascular
channel and transported apically. The rose was taken from the solution and
rinsed in water. The outer bark, cortex, and phloem of the bottom part of the
stem were then gently peeled off, exposing dark continuous lines along
individual 20- to 100-μm-wide xylem channels (Fig. 2).
In some cases, these “wires” extended >5 cm along the stem. From optical and
scanning electron microscopy images of fresh and freeze-dried stems, we
conclude that the PEDOT-S:H formed sufficiently homogeneously ordered hydrogel
wires occupying the xylem tubular channel over a long range. PEDOT-S:H is known
to form hydrogels in aqueous-rich environments, in particular in the presence
of divalent cations, and we assume that this is also the case for the wires
established along the xylem channels of rose stems. The conductivity of
PEDOT-S:H wires was measured using two Au probes applied into individual
PEDOT-S:H xylem wires along the stem (Fig. 3A).
From the linear fit of resistance versus distance between the contacts, we
found electronic conductivity to be 0.13 S/cm with contact resistance being ~10
kilohm (Fig.
3B). To form a hydrogel-like and continuous wire along the inner surface
and volume of a tubular structure, such as a xylem channel, by exposing only
its tiny inlet to a solution, we must rely on a subtle thermodynamic balance of
transport and kinetics. The favorability of generating the initial monolayer
along the inner wall of the xylem, along with the subsequent reduction in free
energy of PEDOT-S:H upon formation of a continuous hydrogel, must be in proper
balance with respect to the unidirectional flow, entropy, and diffusion
properties of the solution in the xylem. Initially, we explored an array of
different conducting polymer systems to generate wires along the rose stems
(table S1). We observed either clogging of the materials already at the inlet
or no adsorption of the conducting material along the xylem whatsoever. On the
basis of these cases, we conclude that the balance between transport,
thermodynamics, and kinetics does not favor the formation of wires inside xylem
vessels. In addition, we attempted in situ chemical or electrochemical
polymerization of various monomers [for example, pyrrole, aniline, EDOT
(3,4-ethylenedioxythiophene), and derivatives] inside the plant. For chemical
polymerization, we administered the monomer solution to the plant, followed by
the oxidant solution. Although some wire fragments were formed, the oxidant
solution had a strong toxic effect. For electrochemical polymerization, we
observed successful formation of conductors only in proximity to the electrode.
PEDOT-S:H was the only candidate that formed extended continuous wires along
the xylem channels.
Fig. 2 Electronically
conducting xylem wires.
(A)
Forming PEDOT-S:H wires in the xylem. A cut rose is immersed in PEDOT-S:H
aqueous solution, and PEDOT-S:H is taken up and self-organizes along the xylem
forming conducting wires. The optical micrographs show the wires 1 and 30 mm
above the bottom of the stem (bark and phloem were peeled off to reveal the
xylem). (B) Scanning electron microscopy (SEM) image of the cross
section of a freeze-dried rose stem showing the xylem (1 to 5) filled with
PEDOT-S:H. The inset shows the corresponding optical micrograph, where the
filled xylem has the distinctive dark blue color of PEDOT. (C) SEM
images (with corresponding micrograph on the left) of the xylem of a
freeze-dried stem, which shows a hydrogel-like PEDOT-S structure.
Fig. 3 Electrical
characterization of xylem wires.
(A)
Schematic of conductivity measurement using Au probes as contacts. (B) I-V
characteristics of PEDOT-S xylem wires of different lengths: L1
= 2.15 mm, L2 = 0.9 mm, and L3 = 0.17 mm.
The inset shows resistance versus length/area and linear fit, yielding a
conductivity of 0.13 S/cm.
It is known
that the composition of cations is regulated within the xylem; that is,
monovalent cations are expelled from the xylem and exchanged with divalent
cations (24). After
immersing the rose stem into the aqueous solution, dissolved PEDOT-S:H chains
migrated along the xylem channels, primarily driven by the upward
cohesion-tension transportation of water. We hypothesize that a net influx of
divalent cations into the xylem occurred, which then increased the chemical
kinetics for PEDOT-S:H to form a homogeneous and long-range hydrogel conductor
phase along the xylem circuitry. The surprisingly high conductivity (>0.1
S/cm) of these extended PEDOT-S:H wires suggests that swift transport and
distribution of dissolved PEDOT-S:H chains along the xylem preceded the
formation of the actual conductive hydrogel wires.
These
long-range conducting PEDOT-S:H xylem wires, surrounded with cellular domains
including confined electrolytic compartments, are promising components for
developing in situ OECT devices and other electrochemical devices and circuits.
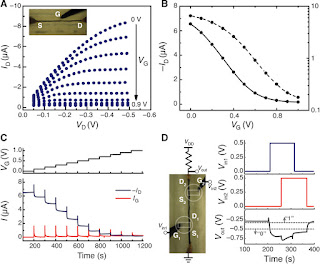
We therefore proceeded to investigate transistor functionality in the xylem
wires. A single PEDOT-S:H xylem wire simultaneously served as the transistor
channel, source, and drain of an OECT. The gate comprised a PEDOT:PSS–coated Au
probe coupled electrolytically through the plant cells and extracellular medium
surrounding the xylem (Fig. 4A,
inset). Two additional Au probes defined the source and the drain contacts. By
applying a positive potential to the gate electrode (VG) with
respect to the grounded source, the number of charge carriers (h+)
in the OECT channel is depleted, via ion exchange (A+) with the
extracellular medium and charge compensation at the gate electrode. This
mechanism defines the principle of operation of the xylem-OECT. The device
exhibited the expected output characteristics of an OECT (Fig. 4A).
Electronic drain current (ID) saturation is also seen, which
is caused by pinch-off within the channel near the drain electrode. Figure 4B
shows the transfer curve, and Fig. 4C
shows the temporal evolution of ID and the gate current (IG)
with increasing VG. From these measurements, we calculate an ID
on/off ratio of ~40, a transconductance (ΔID/ΔVG)
reaching 14 μS at VG = 0.3 V, and very little current leakage
from the gate into the channel and drain (∂ID/∂IG
> 100 at VG = 0.1 V).
Fig. 4 Xylem
transistors and digital logic.
(A)
Output characteristics of the xylem-OECT. The inset shows the xylem wire as
source (S) and drain (D) with gate (G) contacted through the plant tissue. (B)
Transfer curve of a typical xylem-OECT for VD = −0.3 V (solid
line, linear axis; dashed line, log axis). (C) Temporal response of ID
and IG relative to increasing VG. (D)
Logical NOR gate constructed along a single xylem wire. The circuit diagram
indicates the location of the two xylem-OECTs and external connections (compare
with circuit in Fig. 1B).
Voltage traces for Vin1, Vin2, and Vout
illustrate NOR function. The dashed lines on the Vout plot
indicate thresholds for defining logical 0 and 1.
With OECTs
demonstrated, we proceeded to investigate more complex xylem-templated
circuits, namely, xylem logic. Two xylem-OECTs were formed in series by
applying two PEDOT:PSS–coated Au gate probes at different positions along the
same PEDOT-S:H xylem wire. The two OECTs were then connected, via two Au
probes, to an external 800-kilohm resistor connected to a supply voltage (VDD
= −1.5 V) on one side and to an electric ground on the other side (Fig. 4D).
The two gate electrodes defined separate input terminals, whereas the output
terminal coincides with the drain contact of the “top” OECT (that is, the
potential between the external resistor and the OECT). By applying different
combinations of input signals (0 V as digital “0” or +0.5 V as “1”), we
observed NOR logic at the output, in the form of voltage below −0.5 V as “0”
and that above −0.3 V as “1.”
In addition
to xylem and phloem vascular circuitry, leaves comprise the palisade and spongy
mesophyll, sandwiched between thin upper and lower epidermal layers (Fig. 1C).
The spongy mesophyll, distributed along the abaxial side of the leaf, contains
photosynthetically active cells surrounded by the apoplast: the heavily
hydrated space between cell walls essential to several metabolic processes,
such as sucrose transport and gas exchange. Finally, the stomata and their
parenchymal guard cells gate the connection between the surrounding air and the
spongy mesophyll and apoplast, and regulate the important O2-CO2
exchange. Together, these structures and functions of the abaxial side of the
leaf encouraged us to explore the possibility of establishing areal—and
potentially segmented—electrodes in leaves in vivo.
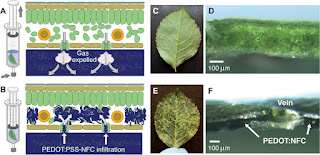
Vacuum
infiltration (25, 26) is a
technique commonly used in plant biology to study metabolite (27) and ion
concentrations in the apoplastic fluid of leaves. We used this technique to “deposit”
PEDOT:PSS, combined with nanofibrillar cellulose (PEDOT:PSS–NFC), into the
apoplast of rose leaves. PEDOT:PSS–NFC is a conformable, self-supporting, and
self-organized electrode system that combines high electronic and ionic
conductivity (28). A rose
leaf was submerged in a syringe containing an aqueous PEDOT:PSS–NFC solution.
The syringe was plunged to remove air and sealed at the nozzle, and the plunger
was then gently pulled to create vacuum (Fig. 5A),
thus forcing air out of the leaf through the stomata. As the syringe returned
to its original position, PEDOT:PSS–NFC was drawn in through the stomata to
reside in the spongy mesophyll (Fig. 5B).
A photograph of a pristine leaf and the microscopy of its cross section (Fig. 5,
C and D) are compared to a leaf infiltrated with PEDOT:PSS–NFC (Fig. 5,
E and F). PEDOT:PSS–NFC appeared to be confined in compartments, along the
abaxial side of the leaf, delineated by the vascular network in the mesophyll (Fig. 5F).
The result was a leaf composed of a two-dimensional (2D) network of
compartments filled—or at least partially filled—with the electronic-ionic
PEDOT:PSS–NFC electrode material. Some compartments appeared darker and some
did not change color at all, suggesting that the amount of PEDOT:PSS–NFC
differed between compartments.
Fig. 5 PEDOT-infused
leaves.
(A)
Vacuum infiltration. Leaf placed in PEDOT:PSS–NFC solution in a syringe with
air removed. The syringe is pulled up, creating negative pressure and causing
the gas inside the spongy mesophyll to be expelled. (B) When the syringe
returns to standard pressure, PEDOT:PSS–NFC is infused through the stomata,
filling the spongy mesophyll between the veins. (C and D)
Photograph of the bottom (C) and cross section (D) of a pristine rose leaf
before infiltration. (E and F) Photograph of the bottom (E) and
cross section (F) of leaf after PEDOT:PSS–NFC infusion.
We proceeded
to investigate the electrochemical properties of this 2D circuit network using
freestanding PEDOT:PSS–NFC films (area, 1 to 2 mm2; thickness, 90
μm; conductivity, ~19 S/cm; ionic charge capacity, ~0.1 F) placed on the
outside of the leaf, providing electrical contacts through the stomata to the
material inside the leaf. We observed typical charging-discharging
characteristics of a two-electrode electrochemical cell while observing clear
electrochromism compartmentalized by the mesophyll vasculature (Fig. 6,
A and B, and movie S1). Upon applying a constant bias, steady-state electrochromic
switching of all active compartments typically took less than 20 s, and the
effect could be maintained over an extended period of time (>10 min).
Likewise, when the voltage was reversed, the observed light-dark pattern was
flipped within 20 s. The electrochromism can be quantified by mapping the
difference in grayscale intensity between the two voltage states (Fig. 6,
C to F). The analysis shows homogeneous electrochromism in the compartments in
direct stomatal contact with the external PEDOT:PSS–NFC electrodes. This is to
be expected, because stomatal contact provides both ionic and electronic
pathways to the external electrodes, allowing continuous electronic charging/discharging
of the PEDOT and subsequent ionic compensation. However, for the intermediate
compartments not in direct stomatal contact with the external electrodes, we
observed electrochromic gradients with the dark-colored side (PEDOT0)
pointing toward the positively biased electrode. This behavior can be explained
by a lack of electronic contact between these compartments—that is, the
infiltrated PEDOT:PSS–NFC did not cross between compartments. As such, these
intermediate compartments operate as bipolar electrodes (29), exhibiting
so-called induced electrochromism (30). Indeed,
the direction of the electrochromic gradients, reflecting the electric
potential gradients inside the electrolyte of each compartment, exactly matches
the expected pattern of induced electrochromism (Fig. 7A).
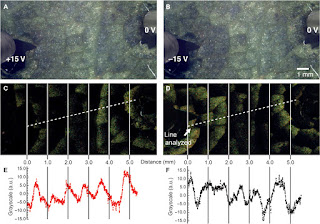
Fig. 6 Electrochromism
in PEDOT:PSS–NFC–infused leaf.
(A
and B) Optical micrographs of the infused leaf upon application of (A)
+15 V and (B) −15 V. Movie S1 shows a video recording of these results. (C
and D) False color map of change in grayscale intensity between
application of (C) +15 V and (D) −15 V. Green represents a positive increase in
grayscale value (light to dark). (E and F) Grayscale values of
pixel intensity along the lines indicated in (C) and (D) showing successive
oxidation/reduction gradients. A plot of the change in grayscale intensity over
a fixed line showing the change and oxidation/reduction gradations versus
distance. a.u., arbitrary unit.
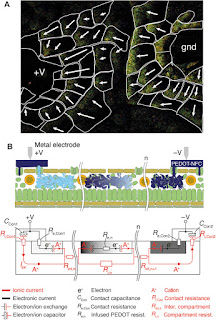
Fig. 7 Leaf
OECD.
(A)
Visualization of the electric field in the leaf-OECD via the induced
electrochromic gradient directions [cf. study by Said et al. (30)]. (B)
Electrical schematic representation of n-compartments modeling both electronic
and ionic components of the current.
In Fig. 7B,
we propose a circuit diagram to describe the impedance characteristics and
current pathways of the leaf-OECD, taking into account both the electronic and
ionic current pathways. The fact that both electrochromic and potential
gradients are established in electronically isolated but ionically connected
areal compartments (Rint,n) along the leaf suggests that the
ionic (Ri,Con) and electronic (Re,Con)
contact resistances across the stomata do not limit the charge transfer and
transport. Although electrochromic switching takes less than 20 s, a constant
current (due to charge compensation and ion exchange) can be maintained for
extended periods of time, suggesting that the capacitance for ion compensation
within the electrodes (CCon) is very large, thus not limiting
the current and transient behavior either. We also found that the induced
electrochromism vanished shortly after the two outer electrodes were grounded,
suggesting that the electronic resistance (Re,n) of the
infused PEDOT:PSS–NFC is lower than that of the parallel ionic resistance (Ri,n).
Our conclusion is that the switch rate of directly and indirectly induced
compartments of the leaf-OECD is limited by ionic—rather than electronic—transport.
The fact
that electrochromically visualized potential gradients are established along
leaf compartments indicates that ion conduction across veins is efficient and
does not limit the overall charge transport. Indeed, we demonstrate above that
induced electrochromism and optical image analysis are powerful tools to
investigate ion migration pathways within a leaf. However, many technological
opportunities and tools require extended electronic conduction along the entire
leaf. Our next target will therefore include development of conductive bridges
that can transport electronic charges across leaf veins as well.
All
experiments on OECT and OECD circuits, in the xylem and in leaves, were carried
out on plant systems where the roots or leaves had been detached from the
plant. In a final experiment, we investigated infusion of PEDOT:PSS–NFC into a
single leaf still attached to a living rose, with maintained root, stem,
branches, and leaves. We found infusion of PEDOT:PSS–NFC to be successful and
we observed OECD switching similar to the isolated leaf experiments
(Supplementary Materials and fig. S1).
Conclusions
Ionic
transport and conductivity are fundamental to plant physiology. However, here
we demonstrate the first example of electronic functionality added to plants
and report integrated organic electronic analog and digital circuits
manufactured in vivo. The vascular circuitry, components, and signals of R.
floribunda plants have been intermixed with those of PEDOT structures. For
xylem wires, we show long-range electronic (hole) conductivity on the order of
0.1 S/cm, transistor modulation, and digital logic function. In the leaf, we
observe field-induced electrochromic gradients suggesting higher hole
conductivity in isolated compartments but higher ionic conductivity across the
whole leaf. Our findings pave the way for new technologies and tools based on
the amalgamation of organic electronics and plants in general. For future
electronic plant technologies, we identify integrated and distributed delivery
and sensor devices as a particularly interesting e-Plant concept for
feedback-regulated control of plant physiology, possibly serving as a
complement to existing molecular genetic techniques used in plant science and
agriculture. Distributed conducting wires and electrodes along the stems and
roots and in the leaves are preludes to electrochemical fuel cells, charge
transport, and storage systems that convert sugar produced from photosynthesis
into electricity, in vivo.
MATERIALS AND METHODS
PEDOT-S wire formation in rose xylem
We used
stems directly cut from a young “Pink Cloud” R. floribunda, with and
without flowers, purchased from a local flower shop. The stems were kept in
water and under refrigeration until they were used for the experiment. The stems
were cleaned with tap water and then a fresh cut was made to the bottom of the
stem with a sterilized scalpel under deionized (DI) water. The stem was then
immersed in PEDOT-S:H (1 mg/ml) in DI water and kept at about 40% humidity and
23°C. Experiments were performed at 70% humidity as well, but no significant
difference was observed during absorption. The rose was kept in the PEDOT-S
solution for about 48 hours. During absorption, fresh 2- to 3-mm cuts to the
bottom of the stem were made every 12 hours. After absorption, the bark and
phloem were peeled off to reveal the xylem. The dissected stem was kept in DI
water under refrigeration until used for characterization and device
fabrication.
Xylem wire device fabrication and characterization
The piece of
stem was mounted on a Petri dish using UHU patafix and was surrounded by DI
water to prevent it from drying out during the experiment. For all the
measurements, Au-plated tungsten probe tips (Signatone SE-TG) with a tip
diameter of 10 μm were used. Using micromanipulators and viewing under a stereo
microscope (Nikon SMZ1500), we brought the probe tips into contact with the
wire and applied a very small amount of pressure for the tips to penetrate the
xylem and make contact with the PEDOT-S inside.
Xylem wire conductivity measurement
Measurements
were performed in the same wire for three different lengths starting from the
longest and then placing one contact closer to the other. We used a Keithley
2602B SourceMeter controlled by a custom LabVIEW program. The voltage was swept
from 0.5 to −0.5 V with a rate of 50 mV/s.
Xylem-OECT construction
The channel,
source, and drain of the OECT are defined by the PEDOT-S wire in the xylem.
Contact with source and drain was made using Au-plated tungsten probe tips. A
PEDOT:PSS [Clevios PH 1000 with 10% ethylene glycol and 1%
3-(glycidyloxypropyl)trimethoxysilane]–coated probe tip was used as the gate.
The tip penetrated the tissue in the vicinity of the channel. All measurements
were performed using a Keithley 2602B SourceMeter controlled by a custom
LabVIEW program.
NOR gate construction
The NOR gate
consisted of two xylem-OECTs and a resistor in series. The two transistors were
based on the same PEDOT-S xylem wire and were defined by two gates
(PEDOT:PSS–coated Au probe tips), placed in different positions near the
PEDOT-S xylem. Using probes, we connected the transistors (xylem wire) to an
external 800-kilohm resistor and a supply voltage (VDD = −1.5
V) on one side and grounded them on the other side. All measurements were
performed using two Keithley 2600 series SourceMeters that were controlled
using a custom LabVIEW program and one Keithley 2400 SourceMeter controlled
manually.
Preparation of PEDOT:PSS–NFC material
A previously
reported procedure was followed with minor modifications for the preparation of
the PEDOT:PSS–NFC material (28). Briefly,
PEDOT:PSS (Clevios PH 1000, Heraeus) was mixed with dimethyl sulfoxide (Merck
Schuchardt OHG), glycerol (Sigma-Aldrich), and cellulose nanofiber (Innventia,
aqueous solution at 0.59 wt %) in the following (aqueous) ratio:
0.54:0.030:0.0037:0.42, respectively. The mixture was homogenized (VWR VDI 12
Homogenizer) at a speed setting of 3 for 3 min and degassed for 20 min in a
vacuum chamber. To make the dry film electrode, 20 ml of the solution was dried
overnight at 50°C in a plastic dish (5 cm in diameter), resulting in a
thickness of 90 μm.
Leaf infusion and contact
A leaf was
excised from a cut rose stem that was kept in the refrigerator (9°C, 35%
relative humidity). The leaf was washed with DI water and blotted dry. The leaf
was placed in a syringe containing PEDOT:PSS–NFC and then plunged to remove
air. Afterward, the nozzle was sealed with a rubber cap. The plunger was gently
pulled (a difference of 10 ml), thereby creating a vacuum in the syringe. The
plunger was held for 10 s and then slowly returned to its resting position for
an additional 20 s. The process was repeated 10 times. After the 10th
repetition, the leaf rested in the solution for 10 min. The leaf was removed,
rinsed under running DI water, and gently blotted dry. Infusion was evident by
darker green areas on the abaxial side of the leaf surface. As the leaf dried,
the color remained dark, indicating a successful infusion of the material. To
make contact to the leaf, small drops (1 μl) of the PEDOT:PSS–NFC solution were
dispensed on the abaxial side of the leaf. PEDOT:PSS–NFC film electrodes were
placed on top of the drops and were air-dried for about 1 h while the leaf
remained wrapped in moist cloth.
Electrochromic measurements
Metal
electrodes were placed on top of the PEDOT:PSS–NFC film, and optical images
(Nikon SMZ1500) were taken every 2 s. A positive voltage potential was applied
(Keithley 2400), and the current was recorded by a LabVIEW program every 250 ms
for 6 min. The time stamp was correlated with the optical images. The voltage
potential was reversed and the process was repeated. Electrochromic effects
were observed between ±2 and ±15 V.
Image analysis
The optical
images were converted to TIFF (tagged image file format) using the microscope
software NIS-Elements BR, opened in ImageJ, and used without further image
processing. The grayscale pixel intensity (0 to 255) was recorded for pixels
along a straight line (Fig. 6,
C and D) by taking the final image (that is, the image after 6 min) for each
state: V1 = +15 V, V2 = −15 V, and V3
= +15 V. Each respective image for the three states was sampled 10 times and
averaged together. Afterward, those averaged grayscale values were subtracted
from the averaged values of the previous state (that is, V2
from V1, and V3 from V2)
representing the changes due to electrochromism plotted in Fig. 6
(E and F). To observe estimated electric field path between the electrodes, the
final image of the second run (V2) was subtracted from the
final image of the first run (V1) in ImageJ to create a false
color image of the changes shown in Fig. 7A.
Additionally, the final image of the third run (V3) was
subtracted from the second run (V2). The false color images
were increased in brightness and contrast, and noise reduction was applied to
reveal the changes in oxidized and reduced states.
SUPPLEMENTARY MATERIALS
Supplementary
material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/10/e1501136/DC1
Methods
Fig. S1.
PEDOT-infused and electrochromic leaves on living rose.
Table S1.
Summary of materials attempted for conducting xylem wires.
Movie S1.
Video recording of electrochromism in PEDOT:PSS–NFC–infused rose leaf.
This is an
open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial
license, which permits use, distribution, and reproduction in any medium,
so long as the resultant use is not for commercial advantage and
provided the original work is properly cited.
REFERENCES AND NOTES
P. H. Raven, R. F. Evert, S. E. Eichhorn, Biology
of Plants (W. H. Freeman, New York, 2005).
B. Buchanan, W. Gruissem, R. Jones, Biochemistry
& Molecular Biology of Plants (Wiley, Hoboken, NJ, ed. 2, 2015).
- ↵
- J. M. Leger
, Organic electronics: The ions have it. Adv. Mater. 20,
837–841 (2008).
- ↵
- X. Wang,
- B. Shapiro,
- E. Smela
, Visualizing ion currents in conjugated polymers.
Adv. Mater. 16, 1605–1609 (2004).
- ↵
- A. Jonsson,
- Z. Song,
- D. Nilsson,
- B. A. Meyerson,
- D. T. Simon,
- B. Linderoth,
- M. Berggren
, Therapy using implanted organic bioelectronics. Sci.
Adv. 1, e1500039 (2015).
- ↵
- J. Rivnay,
- J. Rivnay,
- P. Leleux,
- M. Ferro,
- M. Sessolo,
- A. Williamson,
- D. A. Koutsouras,
- D. Khodagholy,
- M. Ramuz,
- X. Strakosas,
- R. M. Owens,
- C. Benar,
- J.-M. Badier,
- C. Bernard,
- G. G. Malliaras
, High-performance transistors for bioelectronics
through tuning of channel thickness. Sci. Adv. 1, e1400251 (2015).
- ↵
- L. Ouyang,
- C. L. Shaw,
- C. C. Kuo,
- A. L. Griffin,
- D. C. Martin
, In vivo polymerization of
poly(3,4-ethylenedioxythiophene) in the living rat hippocampus does not cause a
significant loss of performance in a delayed alternation task. J. Neural Eng. 11,
026005 (2014).
- ↵
- M. Hamedi,
- A. Elfwing,
- R. Gabrielsson,
- O. Inganäs
, Electronic polymers and DNA self-assembled in
nanowire transistors. Small 9, 363–368 (2012).
- ↵
- L. Groenendaal,
- F. Jonas,
- D. Freitag,
- H. Pielartzik,
- J. R. Reynolds
, Poly(3,4-ethylenedioxythiophene) and its
derivatives: Past, present, and future. Adv. Mater. 12, 481–494 (2000).
- ↵
- R. H. Karlsson,
- A. Herland,
- M. Hamedi,
- J. A. Wigenius,
- R. Åslund,
- X. Liu,
- M. Fahlman,
- O. Inganäs,
- P. Konradsson
, Iron-catalyzed polymerization of
alkoxysulfonate-functionalized 3,4-ethylenedioxythiophene gives water-soluble
poly(3,4-ethylenedioxythiophene) of high conductivity. Chem. Mater. 21, 1815–1821
(2009).
- ↵
- E. Stavrinidou,
- P. Leleux,
- H. Rajaona,
- D. Khodagholy,
- J. Rivnay,
- M. Lindau,
- S. Sanaur,
- G. G. Malliaras
, Direct measurement of ion mobility in a conducting
polymer. Adv. Mater. 25, 4488–4493 (2013).
- ↵
- D. Nilsson,
- M. Chen,
- T. Kugler,
- T. Remonen,
- M. Armgarth,
- M. Berggren
, Bi-stable and dynamic current modulation in
electrochemical organic transistors. Adv. Mater. 14, 51–54 (2002).
- ↵
- J. Bobacka
, Potential stability of all-solid-state ion-selective
electrodes using conducting polymers as ion-to-electron transducers. Anal.
Chem. 71, 4932–4937 (1999).
- ↵
- F. Louwet
, The organic alternative to ITO. Dig. Tech. Pap. Soc.
Inf. Disp. Int. Symp. 39, 663–664 (2008).
- ↵
- S. Ghosh,
- O. Inganäs
, Networks of electron-conducting polymer in matrices
of ion-conducting polymers. Applications to fast electrodes. Electrochem.
Solid-State Lett. 3, 213–215 (2000).
- ↵
- B. Winther-Jensen,
- O. Winther-Jensen,
- M. Forsyth,
- D. R. MacFarlane
, High rates of oxygen reduction over a vapor
phase-polymerized PEDOT electrode. Science 321, 671–674 (2008).
- ↵
- P. Andersson,
- D. Nilsson,
- P.-O. Svensson,
- M. Chen,
- A. Malmström,
- T. Remonen,
- T. Kugler,
- M. Berggren
, Active matrix displays based on all-organic
electrochemical smart pixels printed on paper. Adv. Mater. 14, 1460–1464 (2002).
- ↵
- D. Nilsson,
- N. Robinson,
- M. Berggren,
- R. Forchheimer
, Electrochemical logic circuits. Adv. Mater. 17, 353–358
(2005).
- ↵
- E. Masarovičová,
- K. Kráľová
, Metal nanoparticles and plants. Ecol. Chem. Eng. S 20,
9–22 (2013).
- ↵
- J. P. Giraldo,
- M. P. Landry,
- S. M. Faltermeier,
- T. P. McNicholas,
- N. M. Iverson,
- A. A. Boghossian,
- N. F. Reuel,
- A. J. Hilmer,
- F. Sen,
- J. A. Brew,
- M. S. Strano
, Plant nanobionics approach to augment photosynthesis
and biochemical sensing. Nat. Mater. 13, 400–408 (2014).
- ↵
- D. Djikanović,
- A. Kalauzi,
- M. Jeremić,
- J. Xu,
- M. Mićić,
- J. D. Whyte,
- R. M. Leblanc,
- K. Radotić
, Interaction of the CdSe quantum dots with plant cell
walls. Colloids Surf. B Biointerfaces 91, 41–47 (2012).
- ↵
- H. I. Hussain,
- Z. Yi,
- J. Rookes,
- L. X. Kong,
- D. M. Cahill
, Mesoporous silica nanoparticles as a biomolecule
delivery vehicle in plants. J. Nanopart. Res. 15, 1676 (2013).
- ↵
- P. Kanhed,
- S. Birla,
- S. Gaikwad,
- A. Gade,
- A. B. Seabra,
- O. Rubilar,
- N. Duran,
- M. Rai
, In vitro antifungal efficacy of copper nanoparticles
against selected crop pathogenic fungi. Mater. Lett. 115, 13–17 (2014).
- ↵
- D. M. Miller
, Studies of root function in Zea mays: III. Xylem sap
composition at maximum root pressure provides evidence of active transport into
the xylem and a measurement of the reflection coefficient of the root. Plant
Physiol. 77, 162–167 (1985).
- ↵
- L. Bernstein
, Method for determining solutes in the cell walls of
leaves. Plant Physiol. 47, 361–365 (1971).
- ↵
- B. M. O’Leary,
- A. Rico,
- S. McCraw,
- H. N. Fones,
- G. M. Preston
, The infiltration-centrifugation technique for
extraction of apoplastic fluid from plant leaves using Phaseolus vulgaris as an
example. J. Vis. Exp. 94, e52113 (2014).
- ↵
- G. Lohaus,
- K. Pennewiss,
- B. Sattelmacher,
- M. Hussmann,
- K. Hermann Muehling
, Is the infiltration-centrifugation technique
appropriate for the isolation of apoplastic fluid? A critical evaluation with
different plant species. Physiol. Plant. 111, 457–465 (2001).
- ↵
- J. Kawahara,
- P. A. Ersman,
- X. Wang,
- G. Gustafsson,
- H. Granberg,
- M. Berggren
, Reconfigurable sticker label electronics
manufactured from nanofibrillated cellulose-based self-adhesive organic
electronic materials. Org. Electron. 14, 3061–3069 (2013).
- ↵
- S. Ramakrishnan,
- C. Shannon
, Display of solid-state materials using bipolar
electrochemistry. Langmuir 26, 4602–4606 (2010).
- ↵
- E. Said,
- N. D. Robinson,
- D. Nilsson,
- P.-O. Svensson,
- M. Berggren
, Visualizing the electric field in electrolytes using
electrochromism from a conjugated polymer. Electrochem. Solid-State Lett. 8, H12–H16
(2005).
- ↵
- K. M. Persson,
- R. Karlsson,
- K. Svennersten,
- S. Löffler,
- E. W. H. Jager,
- A. Richter-Dahlfors,
- P. Konradsson,
- M. Berggren
, Electronic control of cell detachment using a
self-doped conducting polymer. Adv. Mater. 23, 4403–4408 (2011).
- R. Gabrielsson, thesis, Linköping University (2012).
- T. A. Skotheim, J. Reynolds, Conjugated Polymers: Theory, Synthesis, Properties, and Characterization (CRC Press, Boca Raton, FL, 2006).
- ↵
- H. E. Gottlieb,
- V. Kotlyar,
- A. Nudelman
, NMR chemical shifts of common laboratory solvents as
trace impurities. J. Org. Chem. 62, 7512–7515 (1997).
Acknowledgments:
We thank M.
Grebe and D. Poxson for help in initiating the project, R. Forccheimmer for
assistance with circuit analysis, A. Malti and J. Edberg for assistance with
the NFC material, and D. Khodagholy and I.-A. Apolozan for assistance with the
LabVIEW programs. Funding: This project was funded primarily by a Knut
and Alice Wallenberg Foundation Scholar grant to M.B. (KAW 2012.0302).
Additional funding was provided by Linköping University and the Önnesjö
Foundation. Author contributions: E.S. tested materials for developing
conducting xylem wires; performed electrical characterization, optical
microscopy, and SEM of the PEDOT-S:H wires; developed the OECT and NOR logic
gate; and analyzed all corresponding data. R.G. synthesized and tested
materials for developing conducting xylem wires. E.G. and E.S. developed the
leaf-OECD. E.G. performed optical microscopy, electrochromic measurements,
image analysis, and the in vivo experiment of leaf-OECD, and designed all
figure illustrations. M.B., E.G., D.T.S., and E.S. developed the electrical
representation of the leaf-OECD. All authors contributed to the initial draft.
M.B. and D.T.S. wrote the final manuscript. O.N. was responsible for the plant
physiology relevance. M.B., D.T.S., X.C., and O.N. supervised the project. M.B.
conceived the project. Competing interests: The authors declare that
they have no competing interests. Data and materials availability: All
data needed to evaluate the conclusions in the paper are present in the paper
and/or the Supplementary Materials. Additional data related to this paper may
be requested from the authors. The data are available upon request to E.S. and
E.G., and the materials are available upon request to R.G.